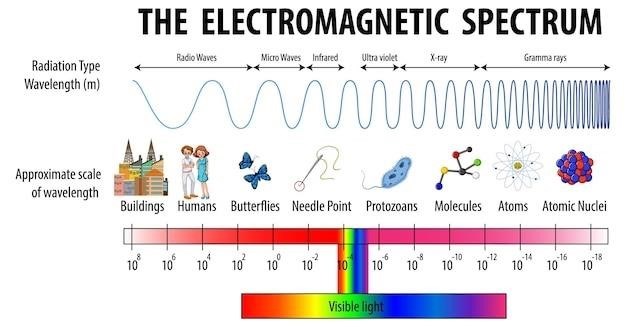
Mass Spectrograph⁚ A Comprehensive Overview
Mass spectrometry analyzes samples by ionizing them, separating ions based on their mass-to-charge ratio, and detecting their abundance․ This powerful technique finds widespread use in various scientific fields, providing detailed compositional information about substances․
Basic Principles of Mass Spectrography
Mass spectrography hinges on the fundamental principle of separating ions based on their mass-to-charge ratio (m/z)․ This separation is achieved by subjecting ions to electric and/or magnetic fields․ Ions with lower m/z values are deflected more readily than those with higher m/z values, allowing for their separation․ The process begins with the ionization of the sample, transforming neutral molecules into charged ions․ Various ionization techniques exist, each suited for different sample types․ Once ionized, the ions are accelerated and passed through a mass analyzer, which separates them according to their m/z․ The separated ions then reach a detector, which measures their abundance․ The resulting data is displayed as a mass spectrum, a plot of ion abundance versus m/z․ This spectrum provides qualitative and quantitative information about the sample composition․ The precise m/z values help identify the different components, while the ion abundances indicate their relative quantities․ Accurate mass measurement allows for precise elemental composition determination, crucial for various analytical applications․
Instrumentation⁚ Ion Source, Analyzer, and Detector
A mass spectrometer comprises three essential components⁚ the ion source, the mass analyzer, and the detector․ The ion source initiates the process by ionizing the sample, converting neutral atoms or molecules into charged ions․ Several ionization methods exist, including electron ionization (EI), chemical ionization (CI), electrospray ionization (ESI), and matrix-assisted laser desorption/ionization (MALDI), each with its strengths and weaknesses depending on the sample type․ The mass analyzer separates the ions based on their m/z ratio․ Common analyzer types include quadrupole, time-of-flight (TOF), and magnetic sector analyzers․ Each analyzer employs different principles to achieve ion separation, offering varying levels of resolution and mass range․ Finally, the detector measures the abundance of each separated ion․ Detectors commonly used include electron multipliers and Faraday cups․ The detector signal is then processed to generate the mass spectrum, a graphical representation of the relative abundance of ions as a function of their m/z ratios․ This spectrum provides the crucial data for qualitative and quantitative analysis․
Applications of Mass Spectrography
Mass spectrometry boasts diverse applications, ranging from identifying compounds in organic chemistry and biology to analyzing gases and materials in various industries․ Its versatility makes it an invaluable analytical tool across scientific disciplines․
Organic Chemistry and Biology Applications
In organic chemistry, mass spectrometry plays a crucial role in identifying and characterizing unknown compounds․ By analyzing the fragmentation patterns of molecules, researchers can deduce their structures and compositions․ This is particularly valuable in natural product isolation and identification, where complex mixtures of organic molecules need to be separated and analyzed․ Furthermore, mass spectrometry is instrumental in determining the molecular weights of newly synthesized compounds, verifying their structures and purity․
Within the biological sciences, mass spectrometry has revolutionized proteomics, metabolomics, and lipidomics․ It allows researchers to identify and quantify proteins, metabolites, and lipids within complex biological samples․ This detailed analysis facilitates the study of cellular processes, disease mechanisms, and drug interactions․ Techniques like MALDI-TOF MS (Matrix-Assisted Laser Desorption/Ionization ⎼ Time of Flight Mass Spectrometry) have significantly advanced microbial identification in microbiology․ This capability accelerates diagnoses and aids in epidemiological studies․
Analysis of Gases and Exhaled Breath
Mass spectrometry offers a highly sensitive and specific method for analyzing gaseous mixtures, making it invaluable in various fields․ Environmental monitoring benefits greatly from its ability to detect and quantify trace amounts of pollutants in air samples, contributing to a better understanding of air quality and its impact on health․ Industrial process control also leverages mass spectrometry to monitor gas compositions in real-time, optimizing processes and ensuring product quality․
Breath analysis, a rapidly growing field, utilizes mass spectrometry to identify volatile organic compounds (VOCs) present in exhaled breath․ These VOCs can serve as biomarkers for various diseases, including lung cancer, diabetes, and kidney failure․ The non-invasive nature of breath sampling and the high sensitivity of mass spectrometry make this a promising diagnostic tool․ Further research continues to explore the potential of breath analysis as a cost-effective and convenient screening method for early disease detection․
Applications in Microelectronics and Materials Science
Mass spectrometry plays a crucial role in the microelectronics industry, providing precise compositional analysis of materials used in semiconductor manufacturing․ It helps ensure the purity of silicon wafers, detects trace impurities that could affect device performance, and characterizes thin films used in integrated circuits․ This precise analysis is critical for maintaining high yields and producing reliable electronic components․ Furthermore, mass spectrometry is employed in the development of new materials with specific electronic properties․
In materials science, mass spectrometry aids in the characterization of various materials, from polymers to metals and ceramics․ It helps determine the elemental composition, isotopic ratios, and molecular structure of materials, providing critical information for understanding their properties and behavior․ This is particularly important in fields such as nanotechnology, where the properties of materials are highly dependent on their precise composition and structure at the nanoscale․ Advanced techniques like secondary ion mass spectrometry (SIMS) enable high-resolution depth profiling, allowing researchers to analyze the composition of materials layer by layer․
Advanced Techniques in Mass Spectrometry
Modern mass spectrometry utilizes sophisticated ionization methods and advanced analyzer designs to achieve higher sensitivity, resolution, and mass accuracy, pushing the boundaries of analytical capabilities․
Soft Ionization Methods
Soft ionization techniques are crucial in mass spectrometry for analyzing thermally labile or large biomolecules, which might fragment extensively under harsh ionization conditions․ These methods minimize fragmentation, preserving the analyte’s intact structure for accurate mass determination and structural elucidation․ Electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI) are prominent examples․ ESI involves spraying a solution of the analyte into a highly charged capillary, producing charged droplets that evaporate to yield gas-phase ions․ MALDI employs a matrix compound that absorbs laser energy, transferring it to the analyte molecules for ionization․ The choice of soft ionization method depends on the sample’s properties and the desired analytical outcome․ Gentle ionization ensures the preservation of non-covalent interactions, enabling the analysis of protein complexes and other fragile biomolecules․ Furthermore, soft ionization enhances the detection of labile post-translational modifications vital for understanding protein function and biological processes․ The reduced fragmentation facilitates the identification of intact molecules, significantly improving the accuracy and reliability of mass spectrometry-based analyses․ The development of novel soft ionization techniques continues to broaden the applicability of mass spectrometry in various research areas․
Triple Focusing Mass Analyzers
Triple focusing mass analyzers represent a sophisticated advancement in mass spectrometry, offering superior performance in resolving power and sensitivity compared to single- or double-focusing instruments․ These analyzers employ three stages of focusing⁚ electrostatic, magnetic, and another electrostatic field․ The initial electrostatic analyzer focuses ions based on their kinetic energy, while the magnetic sector disperses them according to their momentum․ A final electrostatic analyzer corrects for energy spread, resulting in highly precise mass measurements․ This three-stage process significantly improves peak resolution, enabling the differentiation of ions with very similar mass-to-charge ratios․ The enhanced resolution is particularly beneficial for analyzing complex mixtures with closely spaced peaks, allowing the identification and quantification of individual components even in intricate samples․ Triple focusing mass analyzers find applications in various fields, such as elemental analysis, isotope ratio measurements, and the study of complex organic molecules where high mass accuracy and resolution are paramount for accurate structural determination․ The complexity of their design and operation, however, necessitates specialized expertise and sophisticated control systems․
Data Processing and Algorithm Development
Raw data from mass spectrometry experiments is typically complex, requiring sophisticated algorithms for processing and interpretation․ These algorithms address challenges such as noise reduction, peak detection, and mass spectral deconvolution․ Advanced algorithms employ techniques like baseline correction to remove background signals and improve the signal-to-noise ratio, enhancing peak identification․ Peak detection algorithms identify and quantify individual peaks in the mass spectrum, often using sophisticated statistical methods to differentiate real peaks from noise․ Deconvolution algorithms address the issue of overlapping peaks, enabling the separation and quantification of individual components in complex mixtures․ Furthermore, algorithms are crucial for interpreting the data, connecting mass-to-charge ratios to molecular structures, and quantifying the abundance of different components․ This often involves pattern recognition techniques and comparison to spectral libraries․ The development of new algorithms is an active area of research, driven by the need to analyze increasingly complex samples and extract more meaningful information from the data․ Improvements in computational power and machine learning techniques are constantly pushing the boundaries of what’s possible in mass spectrometry data analysis․

Standardization and Quality Control
Reliable mass spectrometry necessitates rigorous standardization and quality control procedures to ensure accurate and reproducible results across different laboratories and instruments․ This includes regular calibration and maintenance checks․
Standardization of Allergenic Extracts
Standardization of allergenic extracts for diagnostic and therapeutic purposes is critical to ensure consistent and reliable results in allergy testing and immunotherapy․ Mass spectrometry plays a crucial role in this process by providing precise and comprehensive characterization of the allergenic components within these extracts․ By identifying and quantifying specific allergens, mass spectrometry helps establish reference standards and quality control metrics․ This detailed characterization enables the development of standardized extracts with consistent potency and composition, minimizing variability between batches and manufacturers․ Furthermore, mass spectrometry aids in the detection of impurities or contaminants that might affect the safety and efficacy of the extract․ This ensures that only high-quality, standardized extracts are used in clinical practice, leading to improved diagnosis and treatment of allergic diseases․ The standardization process involves careful selection and preparation of the allergenic material, followed by rigorous mass spectrometry analysis to ensure consistency and quality control․ This approach promotes accurate diagnosis and effective treatment of allergic conditions, improving patient outcomes and contributing to advancements in allergy research․
Reliability Testing in Mass Spectrometry
Rigorous reliability testing is essential to ensure the accuracy and precision of mass spectrometry results․ This involves evaluating various aspects of the analytical process, from sample preparation and instrument performance to data analysis and interpretation․ Regular calibration and maintenance of the mass spectrometer are crucial, using certified reference materials to verify accuracy and precision․ Internal and external quality control checks are implemented to monitor instrument stability and identify potential systematic errors․ Statistical analysis of replicate measurements helps assess the reproducibility and uncertainty associated with the measurements․ Software validation is also necessary to ensure the reliability of data processing algorithms․ Robustness testing evaluates the performance of the method under varying conditions, such as changes in temperature or sample matrix․ These comprehensive reliability tests are crucial for generating trustworthy and reliable data, ensuring the validity of the conclusions drawn from mass spectrometry experiments across various applications․
Future Directions and Research
Ongoing research in mass spectrometry focuses on enhancing sensitivity, resolution, and speed of analysis․ Miniaturization of mass spectrometers is a key area, leading to portable and field-deployable devices for real-time analysis․ Advanced ionization techniques, like ambient ionization methods, are being developed to analyze samples directly without extensive preparation․ The integration of artificial intelligence and machine learning in data analysis promises to automate data processing, interpretation, and the development of sophisticated algorithms․ Coupling mass spectrometry with other analytical techniques, such as chromatography and electrophoresis, will continue to improve the breadth and depth of information obtained․ Exploring novel mass analyzers and detectors with enhanced performance characteristics is an active area of research․ Furthermore, the development of more robust and user-friendly software is essential for wider accessibility and usability․ These advancements will lead to expanded applications of mass spectrometry in various fields, from medical diagnostics and environmental monitoring to materials science and food safety․